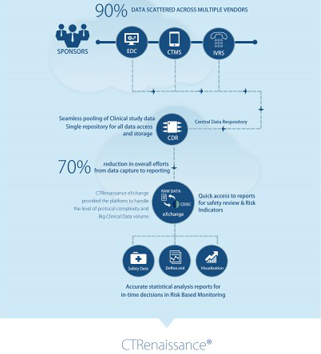
The Business Analyst is part of the Product Strategy team within the Product group helping to guide the current and future business requirements of the MaxisIT CTRenaissance suite of Products as it relates to the internal and external implementation of the product for the company’s business customers.
In this role the Business Analyst understands existing dynamics, situations, and pain points in the Pharmaceutical / Life Sciences / Biotech / Device industries, including Regulatory, Clinical Repositories, Clinical Operations, Clinical Data Reporting, Dashboards, Metrics, Analytics, and Trial Management Reporting.
- Supports the creation of roadmaps
- Participates in Requirements Gathering activities as part of the SDLC across the various functional areas within the Product (EDC, Data
- Management, Biostats, Analytics, Medical Data Review, Drug Safety, Documentation, Regulatory, Submission) etc
- Understand the current trends within the industry to use as a baseline for business requirements
- Ensure business requirements fit together “horizontally across the E2E business functions within the product
- Ensure business requirements are effectively communicated and reach the correct audience
- Liaise as needed with Product Strategy, Development, QA, Testing, and Implementation teams
- Support User Acceptance Test (UAT) activities to ensure that product delivery by the Development Team meets the customer’s needs
- Supports operational delivery and service support as needed
Work with the Quality group to ensure that the enhanced Product fits into the overall MaxisIT Business Processes
- Education Level: Bachelor’s Degree required in Life Science, Informatics, Business or equivalent
- Preference for formal process training (i.e. PMP)At least 3 years of experience in at least 2 areas of the Clinical Trial E2E workflow (Data Capture, Data Management, Biostats, Drug Safety, Health Care, Medical Affairs, Regulatory)
- Proficient knowledge of pharmaceutical Clinical processes & domains
- Proficient knowledge of Clinical Trial phases (I IV)
- Strong technical skills, systems and requirements analysis
- Customer and Service Management focus
- Virtual team experience
- Multicultural and International experience and scope
- Understanding of GxP, CSV and regulations such as 21 CFR Part 11
- Knowledge of industry standards e.g. CDISC, HL7 etc. and applications
- Knowledge of and practical experience of designing clinical data warehouses/repositories / analytics solutions for the pharmaceutical or healthcare industry.
- Experience in Pharma Development and/or management of Clinical Trials function is a plus
Change Management Experience - Proficient knowledge and use of Microsoft Office including Word, Excel, PowerPoint, Visio, and Outlook
- Ability to communicate effectively with Business and Technical staff