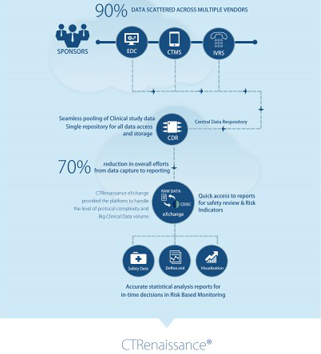
MaxisIT’s Metadata Repository (MDR) holds the definition of data and knowledge from the inside and the outside of an organization. An integral part of the E2E data and workflow streams, MDR can handle standardized metadata under Clinical and Healthcare domains. It is a platform that integrates with multiple 3rd party tools and metadata sources.
 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US