 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US
The world has unwillingly come to a grinding halt, unsure of where to turn, with the impact of the pandemic COVID-19. Even as we submit to social distancing and lockdown requirements based on our geographic location, we are confident that ‘this too shall pass’ and we will resume our lives, ready to pick up where we left off.
In the interim, let’s look at the huge impact this tough situation is having on the conduct of clinical trials. According to this article, clinical trials are set to be severely affected and even the FDA has issued the guidance on the measures needed to manage the anticipated disruptions to the conduct of clinical trials.
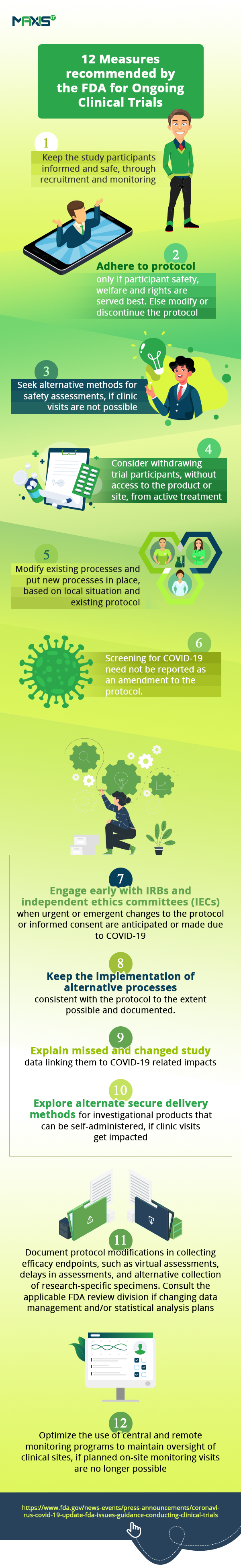
The fall in clinical trial sign-up numbers show us how, even as hospitals are engrossed in helping patients fighting the pandemic, others are unwilling or unable to go anywhere near them. This is affecting the initiation of new trials and the enrollment of new patients into them. Ongoing studies are seeing patient drop-offs. With incomplete visits, we have incomplete data on studies. All these are impacting clinical trial oversight and remote monitoring.
In these challenging times, we need to find new and innovative ways to keep the trials on track and mitigate the risk of having them delayed.
If the situation has in any way affected the information/data being collected, the reason for such disruption and its probable cause will have to be shared with the drug sponsor as well as the regulatory authorities. You may always consider closing the trial a little early and working with data collected till now on an interim analysis to avoid filing for any modifications and deviations and initiate adaptations for trials with additional recruitment later. Protocol changes need to happen quickly while ensuring safety. If some sites have to be closed, we can always adopt a remote approach, without losing control over patient safety, data quality, monitoring of the study and regulatory compliance.
To sum up, it’s time we adapted to our current circumstances and quickly revised the way we manage our trials while remotely maintaining oversight of them. Let’s keep fighting on all fronts, proving the adage that when the going gets tough, the tough keep going.
17 Apr 2019
10 Jun 2019