 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US
Clinical trials must be properly monitored by sponsors, not only to protect the participants in the trials but also to ensure trial data integrity for regulatory compliance. Capable of adapting to the level of risk, Risk Based Quality Management (RBQM) can prove highly effective for onsite-monitoring. This is why sponsors believe RBQM is the way to go when monitoring critical data elements as it helps ensure data quality, improve safety and check the rising costs of drug development.
It’s time to bid goodbye to cumbersome practices which drive up drug development expenses and extend project timelines, with teams traveling to all study sites to check and verify all data points, whether critical or non-critical. Monitoring today can be centralized and remote. All submitted data can be remotely verified to identify missing data trends, outliers and even potential protocol deviations without the need for onsite monitoring. With RBQM, it’s easy to identify even unusual distribution of data at any site, high screen failure or withdrawal rates, high levels of eligibility violations and delays in reporting any critical data elements. Integrated solution providers in RBQM help improve monitoring processes, enabling real-time analysis and improvement in overall quality and compliance.
Evolving RBQM Solutions
An effective, cloud-based integrated solution offers sponsors the ability to gain oversight into clinical investigations by integrating with multiple EDC, CTMS, Safety, Healthcare, PV and document management systems (DMS) and by enabling the standardization and storage of data. The solution’s data analytics capabilities should offer sponsors a range of insights into trial data, including predictive analytics with a dashboard for metrics, key performance indicators, key risk indicators, configurable thresholds, triggers, alerts, escalations, and workflows. This will drive proactive risk mitigation and actionable outcomes and enable timely decision-making. It can also enable course correction throughout the monitoring cycle. Sponsors can now enhance patient safety and data quality, as the solution enables effective protocol design, reduces costs, and offers the ability to strategically adjust oversight in proportion to the level of risk.
Automating and Optimizing Monitoring Activities
Trial monitoring accounts for a substantial portion of total study costs in the drug development cycle and sponsors have explored ways to reduce them through better efficiency in study management. This calls for monitoring functionality that goes over and above the central data collection capabilities made possible by EDC and CTMS systems. With the shift toward risk-based monitoring, technology is helping to collect and track critical data elements, with real-time data standardization and analytics to provide timely insights and shorten trial costs and time significantly. The powerful insights into data powered by modern analytical tools and technologies are driving effective and accurate monitoring and risk mitigation. Modified site management is helping to reduce costs by more than 20%, while improving the quality and integrity of the trial by uncovering problems with the data early, identifying potential risks, and ensuring that the staff at the site are adhering to trial protocols.
Monitoring plans should be tailored to protect trial participants and the integrity of trial data, with focus on preventing or mitigating possible sources of error in the conduct, collection, and reporting of critical data and processes. Sponsors must also analyze ongoing data to continuously assess and adjust their monitoring strategies and activities.
Requirements for RBQM Success
When choosing a solution that will support evolving requirements, ensure that it offers you the following capabilities:
MaxisIT’s own fit-for-purpose RBQM solution offers all these and much more.
About MaxisIT
At MaxisIT, we clearly understand strategic priorities within clinical R&D, as we implement solutions for improving Clinical Development Portfolios via an integrated platform-based approach. For over 17 years, MaxisIT’s Clinical Trial Oversight System (CTOS) has been synonymous with timely access to study-specific, standardized and aggregated operational, trial, and patient data, enabling efficient trial oversight. MaxisIT’s platform is a purpose-built solution, which helps the Life Sciences industry by empowering business stakeholders. Our solution optimizes the clinical ecosystem; and enables in-time decision support, continuous monitoring and regulatory compliance, and greater operational efficiency at a measurable rate.
MaxisIT’s Technology Platform helps small to large biopharmaceutical organizations in their need to build a holistic insight mechanism based on authenticated and aggregated data for effective decision making within the planning, conduct, and submission phases of clinical trials. MaxisIT recently spent time speaking with business leaders from some of our client organizations. The exercise was part of our continuing effort to promote dialog, build trusted relationships, and incorporate our customers’ voices into our planning and solution strategies for 2021.
Pharma companies face huge challenges with the variety and volume of data they receive from across their clinical trial portfolios. Core challenges faced in clinical trial management vary from study design optimization to targeted therapies to benefit-risk assessments to cost reduction. Then we have the need for increased access to a core primary asset or data, for the purposes of decision-making, predictive analysis, insight, and strategic planning. Multiple processes and data formats result from individual applications, structured along departmental and functional silos. Insights are insufficient to help anticipate future business opportunities proactively. Operating in a reactive manner and producing pre-defined visualizations is not enough. The need for digitizing the processes was pressing, but change was slow and sporadic.
In the initial days of the pandemic, on-site visits by trial participants stopped as most sites were busy treating COVID-19 patients and couldn’t spare any infrastructure for the conduct of clinical trials. Patients were also unable or unwilling to go to the sites. This has posed major challenges to the continuity of clinical trials, causing many of them to pause their studies. It wasn’t possible to enroll new patients while the enrolled trial participants were unable to fulfil on-site treatments schedules. With the increasing adoption of digitization, many trials moved to adopt virtual/hybrid processes, which enabled the trial participants to continue participating in a trial remotely. The industry quickly turned to patient-centric technologies like eCOA, ePRO, eConsent, Telemedicine, wearables etc. Thereafter, patients are able to effectively use the various cloud-based apps and tools on their phones to get assessed by physicians remotely and to continue their participation in the clinical trials.
The industry needs to ensure that these app-based, digital technologies are effectively integrated into their single-source-of-truth data hub for effective remote-patient-monitoring, remote-trials-monitoring and ultimately ensure that they get to maintain total oversight on their virtual trials, alongside of trials which are partially virtual (in other words, hybrid) and the trials which are still traditional. Now, all these hybrid/virtual trial set ups need the able support of a technology platform not just to enable remote monitoring, but also to provide sponsors/CROs to stay connected and informed in a virtual manner. The importance of picking the right digital tools to achieve desired outcomes cannot be stressed enough, at this stage.
The data collected from patients, whether reported by them or captured through a wearable technology – can be easily integrated by MaxisIT®’s CTOS. As an evangelist in this field, MaxisIT® addresses all these issues and more with a complete end-to-end integrated solution for companies across the Life Sciences / Healthcare Enterprises. Our solution delivers on two critical challenges faced by the business:
A resolution to these challenges lies in adopting an integrated clinical data management platform like MaxisIT®’s ONE Integrated Clinical Development Platform for the biopharmaceutical industry – which acts as a centralized Command Centre for Remote Clinical Data Review and end-to-end Clinical Data Management. Integrates and interoperates across all eClinical Systems and Virtual Patient Data sources via a secure, cloud-based offering – shifting focus to data management as opposed to data integration. As the industry’s most comprehensive, integrated, automated, and intelligent Data Management Platform, it supercharges data flow across data quality management, remote data review, and study data oversight, readily integrates clinical data from virtual/hybrid sources.
The platform integrates data from different applications or systems at different levels, to manage virtual/hybrid trials, by aggregating all the clinical data in real time, across the data silos. It offers timely access to authoritative, standardized, integrated data and enables risk mitigation with efficient trial oversight via remote monitoring. It makes data quality management possible with real-time controls and statistical computing with assured adherence to timelines and compliance requirements. The platform adopts a command centre approach to truly empowers clinical data management and data review teams to maintain quality and integrity of clinical trials’ patient data by offering seamless interoperable and metadata-driven data, providing the following benefits:
MaxisIT®’s Integrated Clinical Development Platform for biopharmaceutical industry adopts a transformational strategy to detect the leading indicators of change in clinical trial management and empowers key decision-makers to derive maximum value from the data collected across their portfolios. Technologically advanced tools like MaxisIT®’s CTOS have been helping the healthcare industry to enjoy complete oversight across the results of their hybrid/virtual trials. They gain total control over their clinical trial portfolio data to take proactive action, improve the outcomes on their clinical studies and to derive better business outcomes.
About MaxisIT
At MaxisIT, we clearly understand strategic priorities within clinical R&D, and we can resonate that well with our similar experiences of implementing solutions for improving Clinical Development Portfolio via an integrated platform-based approach; which delivers timely access to study specific as well as standardized and aggregated clinical trial operations as well as patient data, allows efficient trial oversight via remote monitoring, statistically assessed controls, data quality management, clinical reviews, and statistical computing. Moreover, it provides capabilities for planned vs. actual trending, optimization, as well as for fraud detection and risk-based monitoring. MaxisIT’s Integrated Technology Platform is a purpose-built solution, which helps Pharmaceutical & Life sciences industry by “Empowering Business Stakeholders with Integrated Computing, and Self-service Dashboards in the strategically externalized enterprise environment with major focus on the core clinical operations data as well as clinical information assets; which allows improved control over externalized, CROs and partners driven, clinical ecosystem; and enable in-time decision support, continuous monitoring over regulatory compliance, and greater operational efficiency at a measurable rate”.
Technology adoption and strategic revisions to processes are redefining clinical trials today. Data collection is taking new approaches, with data from wearables and mobile apps complementing the traditional modes of data collection. The challenge here extends beyond data collection, as the collected data from these diverse sources needs to be integrated and analyzed in real-time to arrive at actionable insights that enable informed decisions.
The time has come for clinical trial sponsors to recognize the critical importance of data collection, aggregation, and integration in managing their trial portfolio. They also need to ensure that the date from trials is standardized and doesn’t demonstrate any variations. Analyzing data for insights when it is not homogeneous could affect the efficiency of a trial and derail its timelines as well as its cost-effectiveness. On the other hand, the ability to source data from disparate sources and integrate it into a single-source-of-truth could offer advantages that include less time, cost, and resources in the conduct of the trial.
We don’t need the COVID-19 pandemic to make us realize that speed is of the essence in any research endeavor like a clinical trial. AI-powered data analytics are able to cut through the time and cost of deriving actionable insights using clinical trial data. Pharma companies are set to reap the benefits of having its drug development teams work in tandem with their data teams when they adopt an AI-powered clinical trial oversight system, which enables real-time coordination to bring in unprecedented efficiencies. When the data from disparate sources gets integrated into a single-source-of-truth, clinical teams get to make informed, data-driven decisions that accelerate their drug development process significantly.
CROs and pharma companies reap the benefits offered by aggregating and analyzing the data collected from clinical trials to enjoy the insights. They also have an opportunity to make timely decisions with regard to the conduct of their trials and to reap the benefits of innovation in many different ways. The global pharma market today fosters a tremendous amount of competition between the players and the time-to-market is of crucial importance to one’s winning strategy as it offers access to superior revenues to a first mover.
About MaxisIT:
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI-enabled analytics platform from data ingestion, processing, analysis to in-time clinical intelligence by establishing the value of data, improve efficiency and empower clinical stakeholders to mitigate risks or seize the opportunity. The CTOS platform helps clinical operations, clinical data management, biostatistics, and clinical R&D portfolio management by bringing clinical operations and patient data together in a single, central data hub (i.e. single-source of truth). The platform allows self-service analytics and role-based clinical intelligence enabling insights, time & cost efficiency, risk mitigation, and the effective management of the portfolio, data quality, patient safety, CRO/site performance management. In short, it helps you maintain an ongoing health-check on your portfolio of clinical trials, using real-time data, analytics, and visualization to drive rigorous analysis of the entire dataset, allowing for proactive trial risk management.
We simplify the monitoring process, feeding all data through a single repository, running robust analytics, and ultimately producing visualizations that are fit for human consumption. Because, yes, complex data analysis can produce simple insights. Real-time data ingestion, analytics, and visualization empower researchers to identify errors as they occur.
The global pharmaceutical industry was pegged by some studies to reach USD 1.57 trillion by 2023, based on some market drivers, growth trends and current and upcoming challenges. Meeting these growth predictions will require the industry to master the management of clinical trials and plug the loopholes which were exposed only too well, by the current pandemic situation around the world. This question will need to be answered, even as the industry is focused on fighting the deadly virus and its spread.
As organizations focus on progressing further with the various clinical trials, which are as necessary as ever, there’s a need to move away from many of the earlier processes which required physical access to patients. Thousands of suspended clinical trials can be resumed with a little tweaking of their original operational models provided of course, there’s adoption of suitable technology. The realization that ‘hybrid’ or virtual trials help to reduce costs, increase patient engagement, improve patient experience and compliance has significantly increased the interest in these models.
All these considerations and technology developments are giving rise to interesting trends in patient engagement, healthcare delivery as well as clinical trial management.
Mobile devices: Mobile devices are helping healthcare providers to keep patients informed and engaged. They also help to measure and collect health-related information like blood pressure levels.
Decentralized trials: Sponsors will have to respect the need of trial subjects to stay safe in place and send the supplies and resources needed to participate in a trial to their doorsteps. Technology is set to play a huge role in enabling such virtual trials.
Wearables and Implants: These smart electronic devices with micro-controllers are seen to be useful to track a person’s fitness and health parameters, till recently. Now they are seen to be reducing the costs for pharma companies and CROs by allowing them to collect clinical trial data, even as the wearer goes about their daily life. Few visits, and no in-clinic monitoring with remote data transmission and collection using technology eliminating any errors in manual collections and calculations! There’s absolutely no downside to this, once the compliance with using the medical data is taken care of.
Personalized medicines: Riding high on the FDA’s commitment to accelerate personalized medicine, pharma products in oncology and neuroscience are especially revolutionizing the go-to-market model of medicine delivery to change healthcare delivery drastically with minimally priced therapies in the coming years.
AI and ML: Accessing an integrated view of the entire trial data for clinical trial oversight and actionable insights using AI-powered technologies with machine learning capabilities is set to revolutionize the design of drugs as well as the conduct of clinical trials to make hyper targeted drugs and precision medicines available.
Cloud computing: The cost savings, competitive advantages and superior efficiencies driven by cloud computing are highly attractive. There’s also a need for better security and lower technology maintenance costs, to which more pharma companies are being drawn.
Pharma as an industry is constantly affected by emerging healthcare needs and rising customer expectations. The need for ongoing research into better treatment options is a constant driver to the industry’s continual research efforts. Rare and orphan diseases are also adding an impetus to these initiatives in developing suitable drug candidates. With billions of dollars being invested into the industry, there’s a lot at stake in the worldwide pharmaceutical markets. This makes it an imperative for the industry to keep pace with the disruptions caused by technology and the changing times.
About MaxisIT:
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform from data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data, improve efficiency and empower clinical stakeholders to mitigate risks or seize the opportunity. The CTOS platform helps clinical operations, clinical data management, biostatistics, and clinical R&D portfolio management by bringing clinical operations and patient data together in a single, central data hub (i.e. single-source of truth). The platform allows self-service analytics and role-based clinical intelligence enabling insights, time & cost efficiency, risk mitigation and the effective management of portfolio, data quality, patient safety, CRO/site performance management. In short, it helps you maintain an ongoing health-check on your portfolio of clinical trials, using real-time data, analytics and visualization to drive rigorous analysis of the entire dataset, allowing for proactive trial risk management.
We simplify the monitoring process, feeding all data through a single repository, running robust analytics and ultimately producing visualizations that are fit for human consumption. Because, yes, complex data analysis can produce simple insights. Real-time data ingestion, analytics and visualization empower researchers to identify errors as they occur.
Many drug candidates and ongoing research studies into their efficacy received serious setbacks when COVID-19 resulted in the total closure of many trial study sites. There’s no saying when these studies will resume and bring deserving drug candidates to the market, to benefit a number of patients who need them, even today. This disruption to patient visits to trial sites for submitting data or receive drug administration has no end in sight, unless we strike off on a new path and transform our processes.
With global lockdowns, social distancing and closed borders, there’s no doubt that digitization has been a great help to many of us. Pharma companies need to focus on improving outcomes and managing costs when testing the efficacy of their drugs, not just in a randomized clinical trial, but in the market as payors and governments alike are looking for drugs with a demonstrated value before they would consider paying a premium for them. This makes it necessary to pick the right digital tools by evaluating the promised outcomes and their desirability in the scheme of things.
Virtual clinical trials have less site dependence. They depend more on telehealth and telemedicine applications. They are aligned with mobile healthcare providers and do not require the trial subjects to visit any site. The conduct all tests virtually, or at some local laboratories or mobile facilities. All consultations can happen over text messages, or audio/video calls. Trial subjects can also get the drugs and delivery devices needed shipped to their addresses, with virtual supervision and nurse assistance being offered 24×7 as support. Data gets collected virtually or through mobile healthcare providers.
Many drug sponsors are becoming suddenly more interested in participating in the digital revolution happening in pharmaceuticals and healthcare are looking for the winning strategy. How assured is digital success? How are we to choose the right initiative? The answer to such questions is not far to seek.
In these highly turbulent times, digitization steps in with various solutions to ease our passage, by:
Technology today is also helping to automate some complex decisions, and helping to streamline and improve the business processes to offer superior quality, execution times and efficiency. This is heralding a sea change in clinical trial process, as more and more sponsors are adopting tools which improve the management of their clinical trials, across geographies.
Remember, that the opportunities to adopt digital technologies are many and varied. It’s possible to really adopt digitization only when companies identify the right initiatives, and truly embrace the opportunity, whether using mobile communications, cloud-based tools and apps, advanced analytics or the Internet of Things. Here’s to more efficiency and speed-to-market and ultimate success in all your endeavors.
About MaxisIT:
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform from data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data, improve efficiency and empower clinical stakeholders to mitigate risks or seize the opportunity. The CTOS platform helps clinical operations, clinical data management, biostatistics, and clinical R&D portfolio management by bringing clinical operations and patient data together in a single, central data hub (i.e. single-source of truth). The platform allows self-service analytics and role-based clinical intelligence enabling insights, time & cost efficiency, risk mitigation and the effective management of portfolio, data quality, patient safety, CRO/site performance management. In short, it helps you maintain an ongoing health-check on your portfolio of clinical trials, using real-time data, analytics and visualization to drive rigorous analysis of the entire dataset, allowing for proactive trial risk management.
We simplify the monitoring process, feeding all data through a single repository, running robust analytics and ultimately producing visualizations that are fit for human consumption. Because, yes, complex data analysis can produce simple insights. Real-time data ingestion, analytics and visualization empower researchers to identify errors as they occur.
As the world was brought to an abrupt halt, the importance of adopting digital technologies has become apparent in all spheres of life, especially so for industries. Will the healthcare and pharmaceutical industry is quickly adopt digital technologies, , in these turbulent times, to fulfill their duty of saving lives and to attain their business goals?
Take the case of clinical trials. The FDA’s requires all sponsors to monitor and validate the process of clinical trials to ensure their integrity. Traditionally, this resulted in a whole lot of work for sponsors and CROs alike, as sponsors undertook site visits to inspect the Contract Research Organization’s (CRO’s) site, its facilities, equipment, personnel, while CROs had to maintain processes and regulatory documentation.
In 2011, the FDA issued modified guidance to encourage more remote processes at reduced cost. Nine years later, the pandemic COVID-19 exerted a very strong negative effect on the conduct of with the closure of one-third of trial sites while nearly 77% of ongoing clinical trials in the USA alone, proving that trials are still not being monitored remotely. After this wake-up call, let’s hope that sponsors and sites alike would take advantage of the advanced technologies available to them to make the remote monitoring of a clinical trial not just possible, but also preferable -given how we may all have to veer towards social distancing and avoid travel, as a way of life.
Data point verification to risk-based monitoring, and everything else in between is possible even when your CRO uses an Electronic Data Capture system (EDC) or a Clinical Trial Management System (CTMS). The AI-powered data analytics modules of such technologies enable the identification of outliers in the data at an early stage and make it possible for the sponsor or CRO to identify risks early and mitigate problems with the trial.
Imagine having the ability to achieve everything the traditional methods could, without any need for travel. What’s more, this would not just improve the efficiency and productivity of a clinical trial but would also shorten the drug candidate’s time to market and reduce costs by up to 30% of the cost of the project. The monitoring happens on the cloud, in a secure environment even as the trials take place in multiple global environments. Remote monitoring allows patients the luxury of not having to travel, encouraging patient recruitment numbers and their retention during the course of the trial.
Using technology, we can track trials remotely and transition from site-based conventional models to mixed models or decentralized models which use electronic content forms, wearables, e-diaries and even remote visits. Research has shown how remote methods keep the number of dropouts also significantly lower when compared to traditional models. The ability to meet the trial’s requirement remotely frees up a patient from the burden of making plans to travel, while fighting the effects of a disease. On the other hand, the research teams could expand the scope of their trials to include patients from around the world, transcending physical barriers to ignore factors like gender, race, age, location and even their ability to travel. The ability to continuously monitor clinical data across one’s portfolio in real-time makes it easier to keep the trials on track, mitigate risks and shorten the time to market.
About MaxisIT:
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform from data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data, improve efficiency and empower clinical stakeholders to mitigate risks or seize the opportunity. The CTOS platform helps clinical operations, clinical data management, biostatistics, and clinical R&D portfolio management by bringing clinical operations and patient data together in a single, central data hub (i.e. single-source of truth). The platform allows self-service analytics and role-based clinical intelligence enabling insights, time & cost efficiency, risk mitigation and the effective management of portfolio, data quality, patient safety, CRO/site performance management. In short, it helps you maintain an ongoing health-check on your portfolio of clinical trials, using real-time data, analytics and visualization to drive rigorous analysis of the entire dataset, allowing for proactive trial risk management.
We simplify the monitoring process, feeding all data through a single repository, running robust analytics and ultimately producing visualizations that are fit for human consumption. Because, yes, complex data analysis can produce simple insights. Real-time data ingestion, analytics and visualization empower researchers to identify errors as they occur.
According to the statistics posted on Worldometer, the world has 803,451 active cases, with 39,044 deaths. This makes COVID_19 preparedness of the highest priority now, turning all other activities non-urgent. This pandemic is taxing the healthcare system and its capabilities, in most countries of the world. The havoc being wrought upon the world by COVID-19 is not just economic. Lockdowns and social distancing requirements are redefining life as we know it.
An ongoing quantitative survey conducted by Continuum Clinical against this context showed how clinical research study sites are rapidly becoming more and more concerned about COVID-19-related interruptions to clinical trial recruitment and retention, with US site concern jumping from 25% to 56% – an 124% increase over just 4 days. Clinical trial sites in Europe also indicated a higher level of concern overall, with nearly 80% of sites indicating the pandemic will negatively impact clinical trial enrollment. Patient willingness to participate in new clinical trials has dramatically come down, according to 78% of European sites and 61% of US sites surveyed.
There’s no doubt that, as a fallout of the COVID-19 situation, drug development is being impacted, as it is:
This unprecedented situation makes it necessary for companies continuing to conduct clinical trials to focus on patient safety, protocol modifications, missed/virtual visits and COVID-19 screening procedures. In the foreseeable future, everything from enrollment and patient care to data collection and analysis will need to be reimagined and managed differently. With most regulatory bodies also accepting this state of affairs, companies are now receiving guidelines on managing protocol changes and data collection methods.
Impact on Data Collection:
As hospitals curtail non-essential visits, there will be an impact on collection, data cleanup, and auditing for clinical trial monitoring. There’s no doubt that the inability of patients to submit data personally will have to be factored into all the existing clinical trial management and any new studies, planning to enroll patients. Everyone is bracing for a long-term impact on data collection even for ongoing studies. No one is sure of the impact of COVID-19 itself on the efficacy data or end results of these studies, given the lack of widespread testing for the virus. A geriatric or immunity-compromised patient infected with the virus would affect the standard assumptions made with regard to the risk of mortality when designing another study, if not tested.
Investigators are planning to adopt some of these workaround solutions to these issues with data collection, by conducting study visits by phone or through video conferencing, while making sure that the integrity of study data is not affected and after documenting any and all deviations.
Technology as an Enabler:
What can we do to redeem this situation and bring trials back on track, to bring medicines to patients who need them and save on the costs of such delays? Given that the NIH and FDA have provided guidance conducting clinical trials using alternate methods to study conduct during the COVID-19 pandemic, those of us who have postponed this decision may take this opportunity to emerge stronger by adopting innovative new technologies which ensure that their work can continue unaffected. Many other regulatory bodies (like the MHRA in the United Kingdom and EMA in Europe are also advocating similar modifications to trial protocols.)
The Clinical Trials Oversight System of MaxisIT brings you the ability to support virtual / decentralized trials by providing seamless integrations with the various patient centric technologies, which are currently used in virtual/decentralized trials. Using the CTOS, a clinical trial sponsor can be assured of receiving continuous streams of qualified data and obtain meaningful insight in real-time into a trial’s performance to maintain oversight as well as to take timely decisions. Data collection methods maybe expanded to include audio/video conferencing with the patient. The patient maybe requested to visit an alternative location, including a laboratory nearby.
About MaxisIT
At MaxisIT, we clearly understand strategic priorities within clinical R&D, as they resonate well with our own experience of implementing solutions for improving Clinical Development Portfolio via an integrated platform. An ideal platform delivers timely access to study-specific as well as standardized and aggregated clinical trial operations as well as patient data, and allows efficient trial oversight via remote monitoring, statistically assessed controls, data quality management, clinical reviews, and statistical computing. Moreover, it provides capabilities for planned vs. actual trending, optimization, as well as for fraud detection and risk-based monitoring.
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform. From data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data. The CTOS empowers clinical stakeholders to mitigate risks and seize the opportunity in the most efficient manner at a reduced cost.
The world has unwillingly come to a grinding halt, unsure of where to turn, with the impact of the pandemic COVID-19. Even as we submit to social distancing and lockdown requirements based on our geographic location, we are confident that ‘this too shall pass’ and we will resume our lives, ready to pick up where we left off.
In the interim, let’s look at the huge impact this tough situation is having on the conduct of clinical trials. According to this article, clinical trials are set to be severely affected and even the FDA has issued the guidance on the measures needed to manage the anticipated disruptions to the conduct of clinical trials.
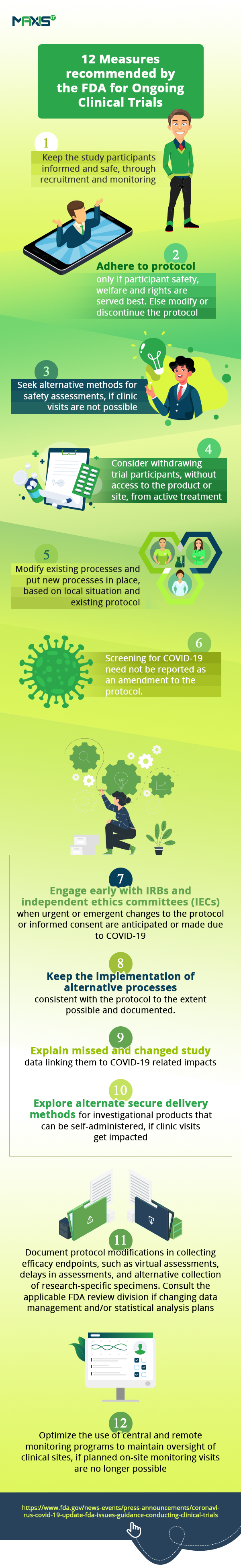
The fall in clinical trial sign-up numbers show us how, even as hospitals are engrossed in helping patients fighting the pandemic, others are unwilling or unable to go anywhere near them. This is affecting the initiation of new trials and the enrollment of new patients into them. Ongoing studies are seeing patient drop-offs. With incomplete visits, we have incomplete data on studies. All these are impacting clinical trial oversight and remote monitoring.
In these challenging times, we need to find new and innovative ways to keep the trials on track and mitigate the risk of having them delayed.
If the situation has in any way affected the information/data being collected, the reason for such disruption and its probable cause will have to be shared with the drug sponsor as well as the regulatory authorities. You may always consider closing the trial a little early and working with data collected till now on an interim analysis to avoid filing for any modifications and deviations and initiate adaptations for trials with additional recruitment later. Protocol changes need to happen quickly while ensuring safety. If some sites have to be closed, we can always adopt a remote approach, without losing control over patient safety, data quality, monitoring of the study and regulatory compliance.
To sum up, it’s time we adapted to our current circumstances and quickly revised the way we manage our trials while remotely maintaining oversight of them. Let’s keep fighting on all fronts, proving the adage that when the going gets tough, the tough keep going.
The world today is caught in a real predicament, living through an apocalyptic scenario, in a daze of disbelief – if not outright denial. This is no fictional account from a Robin Cook bestseller, there isn’t a heroic protagonist stepping up to contain Covid-19. Although we aren’t questioning our chances of survival yet, most aspects of our lives have been seriously altered. As malls, gyms, offices, schools and colleges around us are being closed, work-from-home has become more or less of a slogan. Conferences are getting cancelled or postponed. Most well-laid travel plans and business meetings have come to naught.
What can we do next?
We can simply quarantine ourselves, but not because we are infected. Social distancing is a technique which helps to keep infections at bay, halting the exponential growth of secondary infections mid-stride. Social Distancing keeps the numbers of the ‘infected’ low and ensures that the 5-day incubation period does not make it possible for the ‘exposed’ people go around infecting others in their community, who in turn infect more! To see how this works, follow South Korea’s Patient 31, to whom 1160 people were exposed, and who in turn exposed 9300 others!
This is why everyone is stressing repeatedly on the importance of social distancing today. Doing it tomorrow may prove one day too late for some, while it may be a whole lifetime for others. Let’s not forget that we will never have enough of any of these: hospitals, medical professionals, caregivers, protective gear, ventilators and ICUs to get through a pandemic of this size. More cases will only cause this system to collapse faster. No one is expendable. We cannot choose to discriminate whom to support and whom not to. It becomes a matter of duty – for our personal wellbeing and for the well-being of our communities, to necessarily follow the diktats on social distancing and see that the cases do not grow in number.
Follow Taiwan:
Taiwan was aware that there would be a lot of travel between China and Taiwan around the Lunar New Year. Taiwan took a disaster management approach linking central, regional and local authorities using technology to quickly identify the cases, allot the necessary resources to them while ensuring that they are contained. The country’s national insurance database was integrated with its immigration and customs database to set up alerts on travel and clinical histories together, and tracked their flight origin to determine if they need to be quarantined right away. It offered toll-free numbers in each city for patients to report in, and offered support in every way possible while ensuring that the infection didn’t spread. During this time, it didn’t fail to educate the public and fight misinformation. It took control of its borders, both by air and sea.
What’s next? Mitigation.
Even as we contain the spread of the virus, we must remember that it will not completely vanish. We may slow it down with social distancing but we really need to find ways to mitigate it. In other words, we need to find vaccination against it and a cure for it. Here’s where the Clinical Trials Oversight System would prove useful to you, even when you are keeping a ‘social distance’ and working remotely.
We all know that clinical trials face issues with time and cost overruns and can even fail to clear the hurdles set by regulatory requirements. As you fight these issues, do let us know if you would like to have complementary access to MaxisIT’s CTOS to work with clean data and an AI-powered tool which provides you with actionable insights in real-time.
About MaxisIT
At MaxisIT, we clearly understand strategic priorities within clinical R&D, as they resonate well with our own experience of implementing solutions for improving Clinical Development Portfolio via an integrated platform. An ideal platform delivers timely access to study-specific as well as standardized and aggregated clinical trial operations as well as patient data, and allows efficient trial oversight via remote monitoring, statistically assessed controls, data quality management, clinical reviews, and statistical computing. Moreover, it provides capabilities for planned vs. actual trending, optimization, as well as for fraud detection and risk-based monitoring.
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform. From data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data. The CTOS empowers clinical stakeholders to mitigate risks and seize the opportunity in the most efficient manner at a reduced cost.