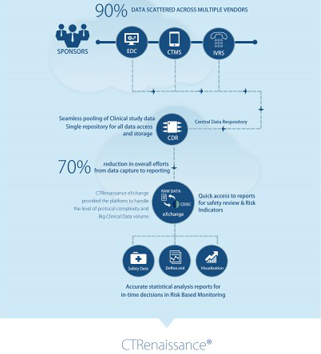
MaxisIT’s cloud-based Risk-based Monitoring (RBM) platform integrated with our CTRenaissance product suite offers an innovative Centralized Monitoring / Risk Based Monitoring solution for the Life Sciences Industry. The platform is based on TransCelerateʼs Risk based Monitoring methodology and is backed by MaxisIT’s knowledgeable and experienced technical/medical/advisory board facilitating a smooth and successful implementation of our solution across various therapeutic areas. The methodology uses Quality Risk Management as a foundation in ensuring subject safety and data quality.
 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US