October 29, 2021
New Jersey, USA – MaxisIT, a leading provider of platform technologies for clinical research, including clinical trial oversight, today announced that they have successfully completed Year 1 of a multi-year agreement with a billion-dollar global biopharmaceutical company focused on developing monoclonal antibody-based therapeutics for the treatment of cancer. The sponsor deployed MaxisIT’s Clinical Trial Oversight System (CTOS) as a strategic part of its digital transformation initiative in under 6 months.
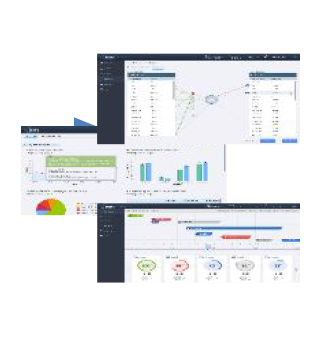
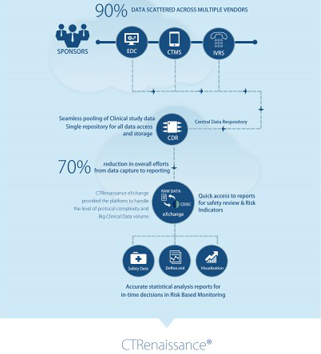
MaxisIT’s cloud-based platform replaced multiple outdated legacy systems offering a single-source-of-truth for clinical, operational, and pre-clinical data. Use of the comprehensive data and analytics platform helped improve the performance of clinical trials, enhance decision making, and reduce costs of the company’s clinical development programs. Timing and accuracy are critical in oncology trials. The CTOS platform is reducing cycle times and providing rapid access to data, offering additional agility to the R&D team. The solution is also being used to automate the complex data ingestion to analytics & visualizations process with 20+ vendor integrations, including digital and real-world data sources.
Oncology trials have become even more complex with the greater adoption of decentralized trials, adaptive trial designs, and new Risk-Based Quality Monitoring guidance. R&D teams need fast access to data to make more meaningful decisions about study designs, protocol compliance, enrollment, CRO performance, and patient safety. Using MaxisIT’s CTOS, the Sponsor’s clinical teams have been accessing and processing an increasing variety and volume of data faster in an automated fashion. The user base has more than doubled in the first year and spans over a dozen clinical trial teams.
“Our clients are recognizing reduced cycle times, increased controls, and improved data quality,“ said Moulik Shah, CEO, MaxisIT. “We are digitally transforming the clinical data supply chain with an omnichannel approach to data and insights in a trial agnostic and eClinical vendor agnostic environment. Our solution is enriching traditional, hybrid, and decentralized trials around the globe.
MaxisIT’s CTOS platform has a comprehensive set of out-of-the-box integrations to most industry-accepted eClinical apps, operational data sources and CROs’ systems. It leverages built-in intelligence that drives automation and reduces manual, error-prone steps in data management, programming, and analytical processes. Overall, it offers greater levels of standardization, reusability, and traceability across siloed clinical data, while truly empowering cross-functional clinical development teams.
Additional Resources
MaxisIT Integrated Clinical Development Platform
MaxisIT Cloud Services Video
MaxisIT Twitter: @MaxisITInc
About MaxisIT
MaxisIT empowers the Biopharmaceutical industry with Integrated Computing, Self-service Analytics, and Enterprise Externalization via its Cloud-based Integrated Clinical Development Platform. For more information about MaxisIT, visit maxisit.com