Sergey Krymgold, Senior Director,
Clinical Trials, Tools and Technologies, Takeda Pharmaceuticals
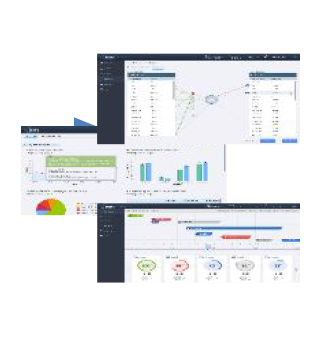
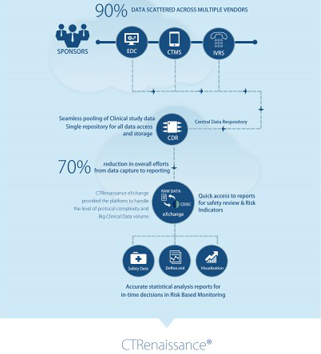
Spearheading innovation in the clinical trials landscape, Sergey Krymgold holds the influential role of Senior Director, Head of Clinical Trials Tools and Technologies organization at Takeda.
With a solid foundation built over 20 years at Biogen, Merck, and within Takeda’s Global Development Organization (GDO), his expertise spans project management, technology development, risk mitigation, and business strategy. As a leader, Sergey drives his organization to transform operational and clinical data into actionable insights, not just navigating the future of digital clinical trials but significantly shaping its direction.
Read More
 Menu
≡
╳
Menu
≡
╳