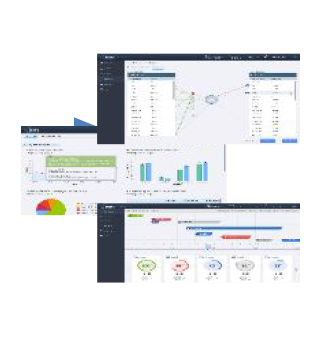
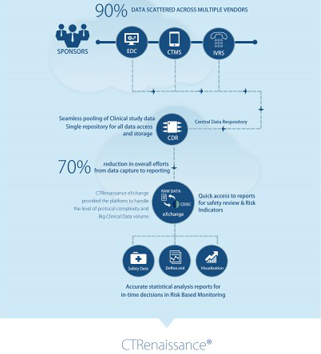
A cloud-based, configurable and homogenous computing platform, Clinical Data Repository (CDR) leverages existing investments by integrating, exchanging and analyzing Health Care data as per industry business needs. It improves accessibility of data in the desired format, empowering end users to effectively utilize data assets. Additionally, CDR serves the industry as a One Stop solution for Data Integration, Aggregation, and Standardization.
 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US