 Menu
≡
╳
Menu
≡
╳
-
WHAT WE DO
- CLINICAL SOLUTIONS
- SERVICES
-
WHO WE ARE
-
RESOURCES
- CAREERS
- CONTACT US
According to the statistics posted on Worldometer, the world has 803,451 active cases, with 39,044 deaths. This makes COVID_19 preparedness of the highest priority now, turning all other activities non-urgent. This pandemic is taxing the healthcare system and its capabilities, in most countries of the world. The havoc being wrought upon the world by COVID-19 is not just economic. Lockdowns and social distancing requirements are redefining life as we know it.
An ongoing quantitative survey conducted by Continuum Clinical against this context showed how clinical research study sites are rapidly becoming more and more concerned about COVID-19-related interruptions to clinical trial recruitment and retention, with US site concern jumping from 25% to 56% – an 124% increase over just 4 days. Clinical trial sites in Europe also indicated a higher level of concern overall, with nearly 80% of sites indicating the pandemic will negatively impact clinical trial enrollment. Patient willingness to participate in new clinical trials has dramatically come down, according to 78% of European sites and 61% of US sites surveyed.
There’s no doubt that, as a fallout of the COVID-19 situation, drug development is being impacted, as it is:
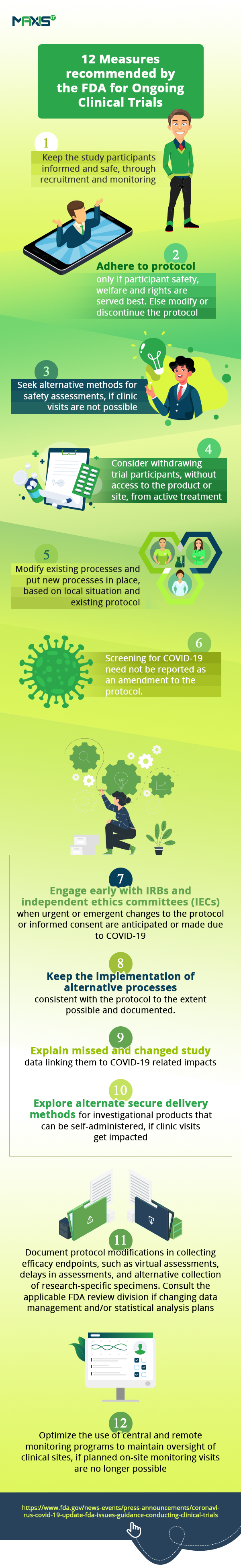
This unprecedented situation makes it necessary for companies continuing to conduct clinical trials to focus on patient safety, protocol modifications, missed/virtual visits and COVID-19 screening procedures. In the foreseeable future, everything from enrollment and patient care to data collection and analysis will need to be reimagined and managed differently. With most regulatory bodies also accepting this state of affairs, companies are now receiving guidelines on managing protocol changes and data collection methods.
Impact on Data Collection:
As hospitals curtail non-essential visits, there will be an impact on collection, data cleanup, and auditing for clinical trial monitoring. There’s no doubt that the inability of patients to submit data personally will have to be factored into all the existing clinical trial management and any new studies, planning to enroll patients. Everyone is bracing for a long-term impact on data collection even for ongoing studies. No one is sure of the impact of COVID-19 itself on the efficacy data or end results of these studies, given the lack of widespread testing for the virus. A geriatric or immunity-compromised patient infected with the virus would affect the standard assumptions made with regard to the risk of mortality when designing another study, if not tested.
Investigators are planning to adopt some of these workaround solutions to these issues with data collection, by conducting study visits by phone or through video conferencing, while making sure that the integrity of study data is not affected and after documenting any and all deviations.
Technology as an Enabler:
What can we do to redeem this situation and bring trials back on track, to bring medicines to patients who need them and save on the costs of such delays? Given that the NIH and FDA have provided guidance conducting clinical trials using alternate methods to study conduct during the COVID-19 pandemic, those of us who have postponed this decision may take this opportunity to emerge stronger by adopting innovative new technologies which ensure that their work can continue unaffected. Many other regulatory bodies (like the MHRA in the United Kingdom and EMA in Europe are also advocating similar modifications to trial protocols.)
The Clinical Trials Oversight System of MaxisIT brings you the ability to support virtual / decentralized trials by providing seamless integrations with the various patient centric technologies, which are currently used in virtual/decentralized trials. Using the CTOS, a clinical trial sponsor can be assured of receiving continuous streams of qualified data and obtain meaningful insight in real-time into a trial’s performance to maintain oversight as well as to take timely decisions. Data collection methods maybe expanded to include audio/video conferencing with the patient. The patient maybe requested to visit an alternative location, including a laboratory nearby.
About MaxisIT
At MaxisIT, we clearly understand strategic priorities within clinical R&D, as they resonate well with our own experience of implementing solutions for improving Clinical Development Portfolio via an integrated platform. An ideal platform delivers timely access to study-specific as well as standardized and aggregated clinical trial operations as well as patient data, and allows efficient trial oversight via remote monitoring, statistically assessed controls, data quality management, clinical reviews, and statistical computing. Moreover, it provides capabilities for planned vs. actual trending, optimization, as well as for fraud detection and risk-based monitoring.
MaxisIT’s Clinical Trials Oversight System (CTOS) enables “data-driven digital transformation” by its complete AI enabled analytics platform. From data ingestion, processing, analysis to in-time clinical intelligence by establishing value of data. The CTOS empowers clinical stakeholders to mitigate risks and seize the opportunity in the most efficient manner at a reduced cost.
The world has unwillingly come to a grinding halt, unsure of where to turn, with the impact of the pandemic COVID-19. Even as we submit to social distancing and lockdown requirements based on our geographic location, we are confident that ‘this too shall pass’ and we will resume our lives, ready to pick up where we left off.
In the interim, let’s look at the huge impact this tough situation is having on the conduct of clinical trials. According to this article, clinical trials are set to be severely affected and even the FDA has issued the guidance on the measures needed to manage the anticipated disruptions to the conduct of clinical trials.
The fall in clinical trial sign-up numbers show us how, even as hospitals are engrossed in helping patients fighting the pandemic, others are unwilling or unable to go anywhere near them. This is affecting the initiation of new trials and the enrollment of new patients into them. Ongoing studies are seeing patient drop-offs. With incomplete visits, we have incomplete data on studies. All these are impacting clinical trial oversight and remote monitoring.
In these challenging times, we need to find new and innovative ways to keep the trials on track and mitigate the risk of having them delayed.
If the situation has in any way affected the information/data being collected, the reason for such disruption and its probable cause will have to be shared with the drug sponsor as well as the regulatory authorities. You may always consider closing the trial a little early and working with data collected till now on an interim analysis to avoid filing for any modifications and deviations and initiate adaptations for trials with additional recruitment later. Protocol changes need to happen quickly while ensuring safety. If some sites have to be closed, we can always adopt a remote approach, without losing control over patient safety, data quality, monitoring of the study and regulatory compliance.
To sum up, it’s time we adapted to our current circumstances and quickly revised the way we manage our trials while remotely maintaining oversight of them. Let’s keep fighting on all fronts, proving the adage that when the going gets tough, the tough keep going.